Abstract
Background
We previously demonstrated that the combination of Nilotinib (NIL) + Pegylated IFN-a2a (Peg-IFN) is able to induce high deep molecular response rates in chronic phase CML (CP CML) patients, as first-line therapy (Nicolini FE et al., Lancet Haematol. 2015).
Aims
Assessment of the molecular responses obtained with the same combination vs NIL alone prospectively, in newly diagnosed CP-CML. (EudraCT 2013-004974-82).
Methods
Patients (pts) ≤65 years with no history of arterial damage were randomized 1:1 to get NIL 300 mg BID alone (M0 to M48, arm A) vs Peg-IFN alone (± HU) for 30 days (M-1 to M0) 30 mg/wk as a priming, prior to a combination of NIL 300 mg BID + Peg-IFN 30 mg/wk 2 weeks, upgraded to 45 mg/wk thereafter if proper tolerance for up to 2 years (M0 to M24, arm B) followed by NIL alone for 2 more years. The primary endpoint was the rate of molecular response 4.5 (MR4.5) by 1 year. Molecular assessments were centralised, quantifications were expressed as BCR-ABL/ABL1 (IS) in % with ≥32,000 copies of ABL1 as control.
Results
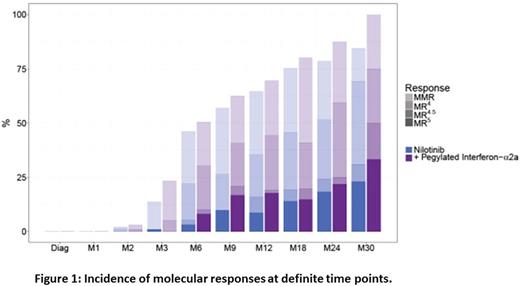
Two hundred and one pts were randomized (100 in arm A, 101 in arm B), 65 males in both arms, 35 females in arm A, 36 in arm B. The median follow-up is 20.6 (9.1-34.7) months. Results are analysed in intention-to-treat. Sokal scores were high in 25%, intermediate in 36% and low in 39% of pts; Euro scores were high in 13%, intermediate in 44% and low in 43% of pts; Eutos LTS scores were high in 2%, intermediate in 17%, and 81% low; equally balanced in the 2 arms The median age was 46 (18-66) years, equally balanced. Eight (4%) pts had a cryptic Philadelphia chromosome, 12 (6%) a variant form, and 15 (7.5%) had ACAs, all pts had a "Major" BCR transcript. CHR was obtained in 9.6% of pts at M0 (arm B) and in 88% of pts in arm A and 90.4% of pts in arm B at M1. The rates of CCyR at M3 were 63% vs 65% in arms A and B, and BCR-ABL1≤1% at M6 were 83% in arm A vs 86% and arm B, on evaluable samples. The incidence of molecular responses are shown in Fig. 1.
Of note, 90% of the pts had a BCR-ABL1 ≤10% at M3 in arm A vs 84% in arm B (p=ns). By M12, the rates of MMR were 69.9% vs 72.4% (p=0.079), MR4 were 34.65% vs 47.9% (p=0.094), MR4.5 were 17.9% vs 24.11% (p=0.272), MR5 12.1% vs 22.31% (p=0.075), in arm A and arm B respectively. Data from 11 pts in arm A and 16 in arm B at M12 are still pending. Definitive results at 1 year will be presented. One pt progressed toward accelerated phase in arm A with a Y253H mutation. Fifteen pts were withdrawn from study in arm A (toxicity 5, other cancer 2, failure 8) and 12 patients from arm B (toxicity 6, failure 6), no pt died. Interestingly, 5 mutated (ie. failure) pts were found in arm A (3 Y253H, 1 E225K, 1 F317L), vs only 1 pt (T315I) in arm B. The median dose of Peg-IFN delivered in arm B during the first month is 30 (0-30) mg/wk, 30 (0-45) mg/wk at M2, 45 (0-45) mg/wk at M3, 37.5 (0-45) mg/wk at M6, 30 (0-45) mg/wk at M9 and 12. The median doses of NIL delivered were 600 mg daily at M2, 3, 6, 9, 12 as initially planned in both arms. The rate of grade 4 hematologic toxicities overall was 15%, with no anemias, 1% and 4% thrombocytopenias, 3% and 4% neutropenias, 0% and 1% leucopenias, and 0 and 1% pancytopenias in arms A and B respectively. Grade ¾ non-hematologic toxicities consisted in 4% of cardiac disorders in arm A (1 coronaropathy, 2 thoracic pains and 1 atrial fibrillation) vs 1% in arm B (palpitation), 2% vascular disorders in arm A (1 pulmonary embolism, 1 transient ischemic attack) and 1% in arm B (PAOD). Three % of gastro-intestinal disorders in arm A (resolutive pancreatitis) vs 1% in arm B (anal fissure); 1% of skin disorders in arm A; 2% auto-immune disorders in arm B (1 recurrent pericarditis, 1 hemolytic anemia); 2 and 5 pregnancies (of the partner except 1) were observed in arm A and B respectively, despite recommended contraceptive methods. We observed 10% lipase elevations in arm A, 3 in arm B, 2% cholestatic episodes in arm A, 1% in arm B; 1% of transaminase elevations in each arm. There were 2% depressive episodes in arm B, 1% in arm A; infections were detected in 1% arm 1 and 3% in arm B. Finally 3 intercurrent cancers were detected in arm A (cervix, breast, thyroid).
Conclusion
The combination of NIL + Peg-IFN seems to provide slightly deeper molecular responses rates (especially MR5) by M12, but so far not significantly, in newly diagnosed CP CML pts without increasing the rate of more frequent early SAEs in such a setting. Definitive results at M12 will be updated for the meeting.
Nicolini: BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; ARIAD: Honoraria, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau. Etienne: Incyte: Speakers Bureau; BMS: Speakers Bureau; Novartis: Consultancy, Research Funding. Guerci-Bresler: BMS: Speakers Bureau; Incyte: Speakers Bureau; Novartis: Speakers Bureau; Pfizer: Speakers Bureau. Charbonnier: Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Legros: BMS: Speakers Bureau; Incyte: Speakers Bureau; Novartis: Speakers Bureau. Coiteux: Incyte: Speakers Bureau; BMS: Speakers Bureau. Cony-Makhoul: BMS: Speakers Bureau. Rousselot: Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sunesis: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Pfizer: Research Funding. Guyotat: BMS: Speakers Bureau. Ianotto: Novartis: Other: Grant. Rea: BMS: Consultancy, Speakers Bureau; Incyte: Speakers Bureau; Novartis: Speakers Bureau; Pfizer: Speakers Bureau. Mahon: Pfizer: Speakers Bureau; Incyte: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Novartis: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal